Power Teams
Magnesium-Air Battery
Project Managers: Rosa Zhang & Matthew Moy
Team Members: Fall: Aaron Lin, Rustam G., Sean F.;
Spring: Aaron Lin, Rustam G., Sean F., Chuying H., Jeffrey M., Lukas B.,Shefali A B.
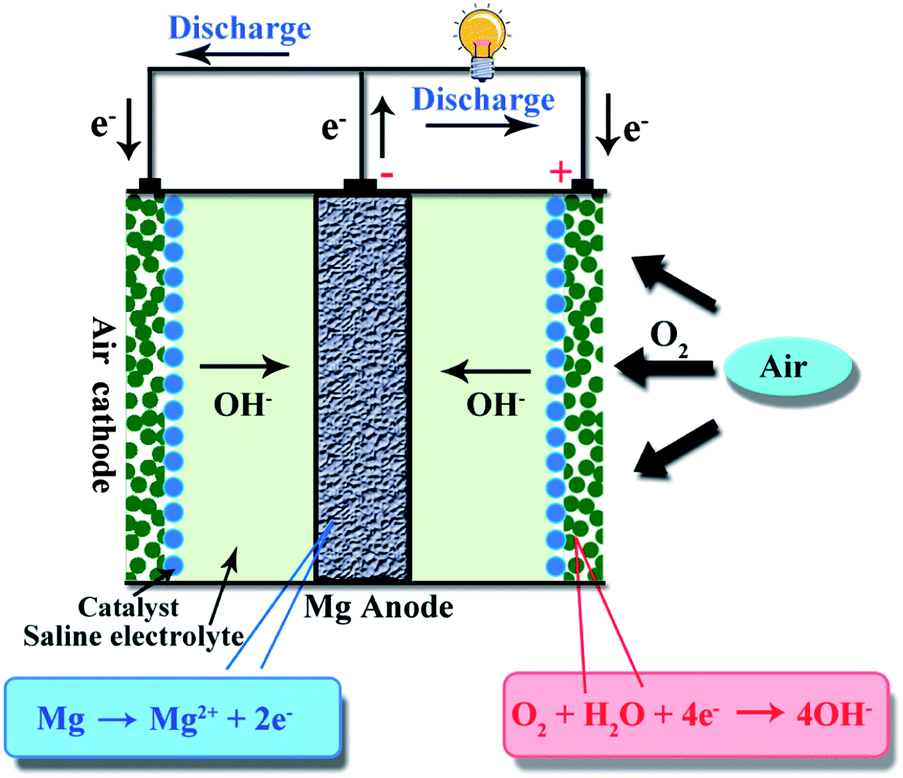 |
|
Lead-Acid Battery
Project Manager: Karl Mattsson
Team Members: Fall: Katherine W., Michael X.;
Spring: Katherine W., Michael X., Ethan Y., Ian L., Quyen L T., Negar B.
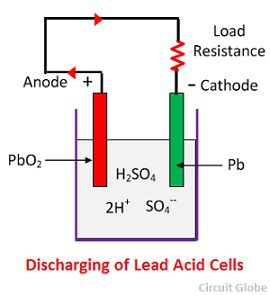 |
We are developing a lead-acid battery to power our car. The chemical energy in a lead-acid battery is stored in the potential difference between the two lead plates (PbSO4 and PbO2), and our goal is to harness this energy to provide power to the motor of our car. Some of the projects we are working on are determining optimal cell arrangement, characterizing energy and power density, and cycling degradation. |
Thermoelectric
Project Manager: Jacob Zamojc
Team Members: Aditya Parekh, Ariana Coracero, Jason Jayalie, Jason Morales, Kishan Aryasomayajula, Vidushi Bansal
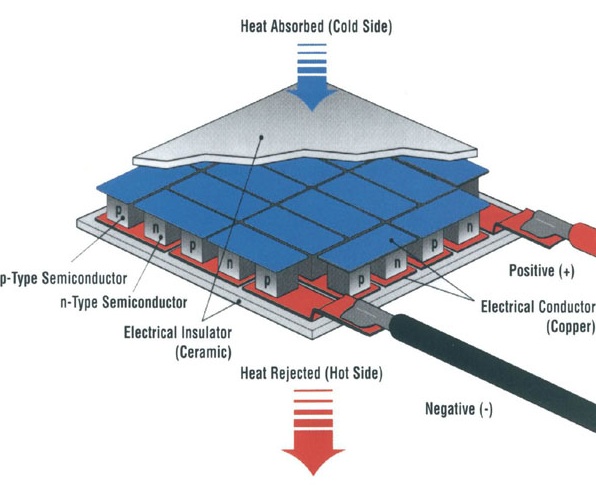 |
The hottest and coolest team in ChemE Car! The Thermoelectric team uses Thermoelectric Generators (TEGs) which produce electric energy when sandwiched between a hot side (boiling water/sulfite reaction) and a cold side (acetone + dry ice). Our current goal is to collect swaths of data to create calibration curves for our design and to investigate novel configurations and optimizations for TEGs. |
Aluminum-Air Battery
Project Managers: Srikant Sangireddy and Edward Hsu
Team Members: Fall: Ariana C., Wudy Y., Chino R.;
Spring: Ariana C., Nyah D., Stephanie G., Joseph M., Akfred V., Ashley W.
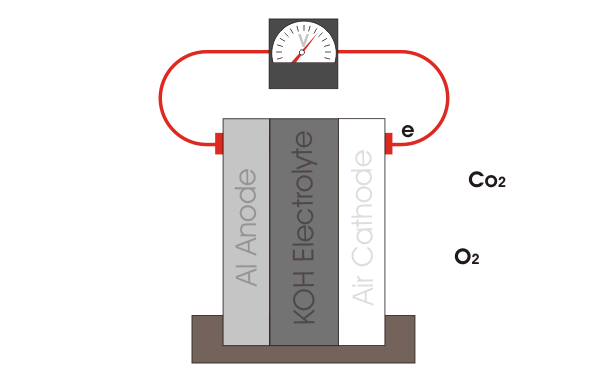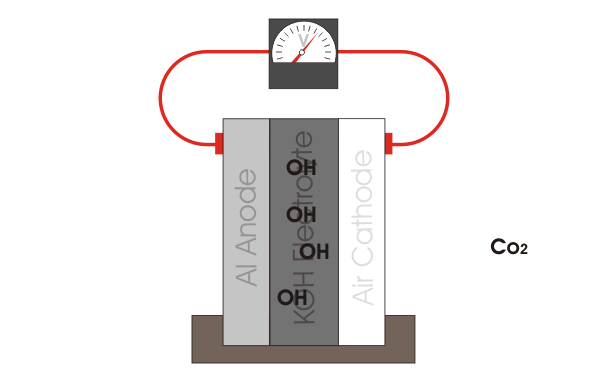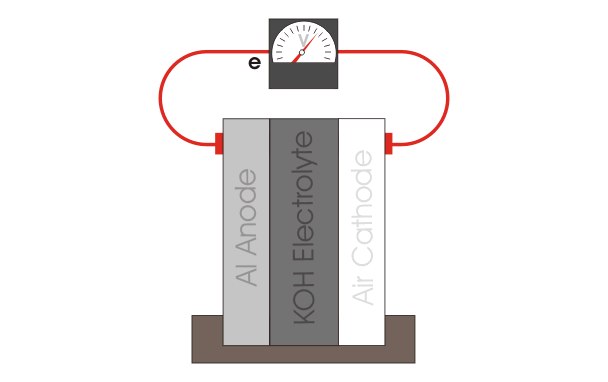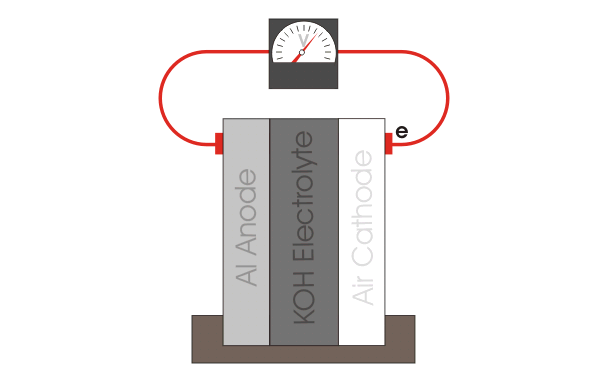 |
The aluminum air battery is a non-rechargeable battery with a very high energy density. The anode consists of an aluminum sheet, which is separated from the cathode, a carbon paste, via filter paper. The whole cell is wetted with KOH electrolyte. Multiple of these cells are stacked up in series to produce a power output large enough to power the car. Our teams current goals are to explore different anode materials, electrolyte additives and optimizing the gas diffusion layer. |
Zinc-Air Battery
Project Managers: Fall: Alexander Yao; Spring: Michelle Zhao & QiWen Li
Team Members: Fall: Michelle Z., QiWen L., Monica W., Juana G.;
Spring: Joshua L., William W., Elena B., Anthony G., Cameron M., Reese C.
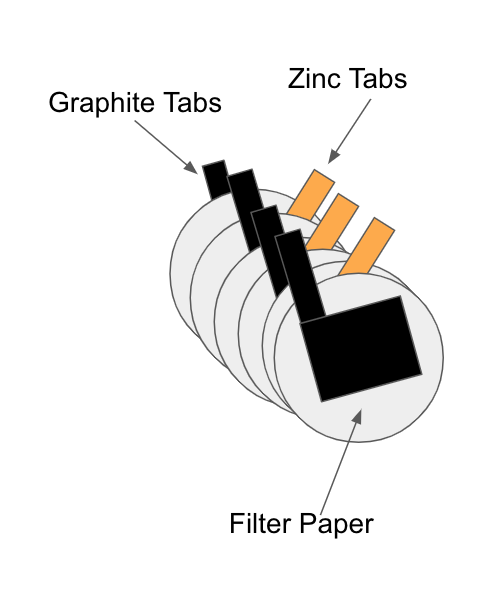 |
The zinc-air battery team has been part of chemE car for several years and performed at both the regional and national competitions. The components of the battery include a zinc anode, a carbon-based cathode that captures oxygen from air, and potassium hydroxide electrolyte. Our main goals for this year include researching and testing new improvements to our battery while focusing on calibration and consistency to excel at competition. |
Fuel Cell Battery
Project Managers: Ashutosh Bhadouria & Rohit Rungta
Team Members: Fall: Aziz F., Sean F., Daniela P. R., Inji P., Alex D., Esther L., Zena, Juan G., Rustam G.;
Spring: Aziz F., Sean F., Daniela P. R., Anderson W., Arya M., Aaron Lin, Pancham P., Pragnay N., Prisha D., Raphael L., Reece Q., Tara D.
 |
Our team uses a novel Fe (VI) salt to replace the MnO2 cathode in conventional (Zn-MnO2) alkaline battery. This 'Super' Iron setup has been reported to have a 25% increase in energy output than its conventional counterpart. Our experimental battery team strives to achieve these results in the lab and create a battery that is chemically superior to the current ones being used on the car. If you want to be involved with hands-on research and be part of a superior battery team- look no further. For questions and concerns, please feel free to reach out to Ashu and Rohit. |
Zinc Carbon/Supercapacitor Battery
Project Manager: Declan Mahaffrey-Dowd
Team Members: Fall: Aziz F., Daniela P. R., Inji P.;
Spring: Aryan N., Kshitij C. (TJ), Jin Y., Alfred V., Reece Q.
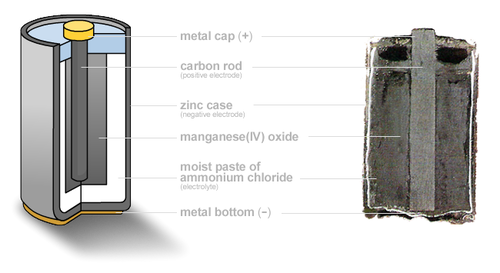 |
The zinc-carbon battery is an old, cheap battery chemistry. This battery is based around the basic reaction Zn + MnO2 → ZnO + Mn2O3, but in reality, the reaction gets much more complicated depending on the electrolyte composition and the discharge level. Our team will be investigating several factors that allow us to optimize the performance of our battery for the intended applications, including electrolyte composition, amounts of zinc corrosion inhibitor added, the carbon to MnO2 ratio in the cathode, and more! |
Control Teams
Sulfur Clock
Project Manager: Purvaansh Lohiya
Team Members: Fall: Jason M., Samantha Y., Ethan N.;
Spring: Jason M., Aaron L., Ben E., Eula B., Jimin J.
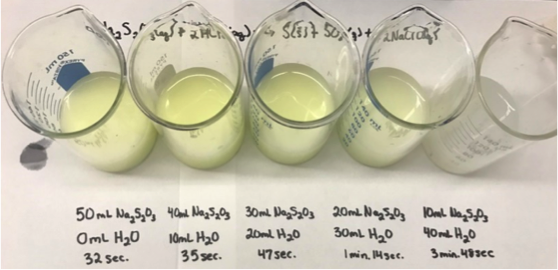 |
A new experimental clock team, Sulfur Clock uses the reaction between sodium thiosulfate and hydrochloric acid to form a sulfur precipate, changing the reacting solution's transparency. While this is one method of experimentation, we will also be testing properties of the reaction. Our goals for the semester are to establish a calibration curve for our reaction, and conduct research on other potential forms of stopping mechanisms. |
Vitamin C Clock
Project Manager: Ethan Nguyen & Samantha Yang
Team Members: Fall: Jason M. Michelle Z., Aaron Liu, Corey;
Spring: Jason M., Amy L., Eula B., Stephen C., Drue H.
 |
A new experimental clock team, Sulfur Clock uses the reaction between sodium thiosulfate and hydrochloric acid to form a sulfur precipate, changing the reacting solution's transparency. While this is one method of experimentation, we will also be testing properties of the reaction. Our goals for the semester are to establish a calibration curve for our reaction, and conduct research on other potential forms of stopping mechanisms. |
Electrochemical Clock
Project Manager: Aditya Parekh & Niharika Gupta
Team Members: Fall: Colby C., Chino R., Chandini D.
Spring: Nicole N., Alaa A., Quinn T., Sidhartha B., Ashley C.
Enzymatic Clock
Project Manager: Chino Ruttanasupagid & Jason Hou
Team Members: Fall: Vidushi B., Aaron Lin, Bonnie Z.;
Spring: Vidushi B., Aaron F., Ethan L., Riley M., Hunter O.
 |
Our team takes advantage of the catalytic decomposition of hydrogen peroxide via the catalase enzyme. This reaction produces oxygen, pushing a viscous fluid through a tube, sending a message to the car to stop. Catalase is one of the fastest and most impressive enzymes known to man, and can be found in almost all living things. We currently blend up spinach as our source of catalase, but hope to look into other sources this year. One advantage of enzymatic clock is that all materials involved are completely safe and environmentally friendly, and thus requires no advanced waste handling. Our goals for the year include improving the reaction aparatus in order to stop leaking, and to create a very accurate calibration curve for the timing of the reaction. |
Chassis Teams
Electrical
Project Managers: Nicole Stokowski
Team Members: Caitlin Iswono, Darby Clement, Mehul Raheja, Zena Quach
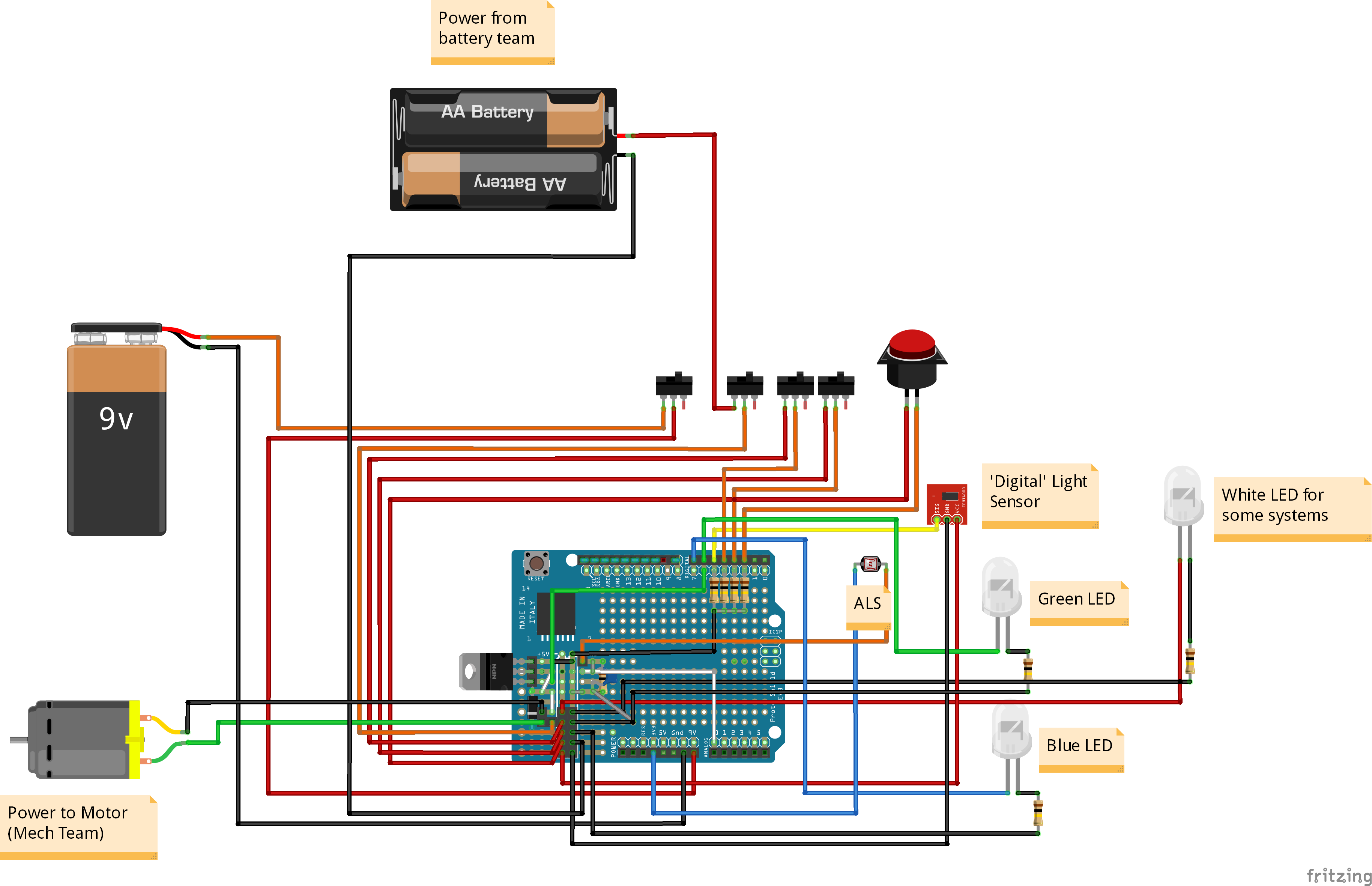 |
The electrical team devises ways of converting the Control Teams’ data into voltages so that we can determine whether the car should be running or not. Using this data, we turn on and off the power supplied by the Power & Battery Teams to the motor. Currently, we are trying to convert to using an Arduino Nano, as well as increasing data collection for the different teams by measuring voltage and current from the batteries as well as measuring the time it takes to go a certain distance to calculate a more accurate velocity. |
